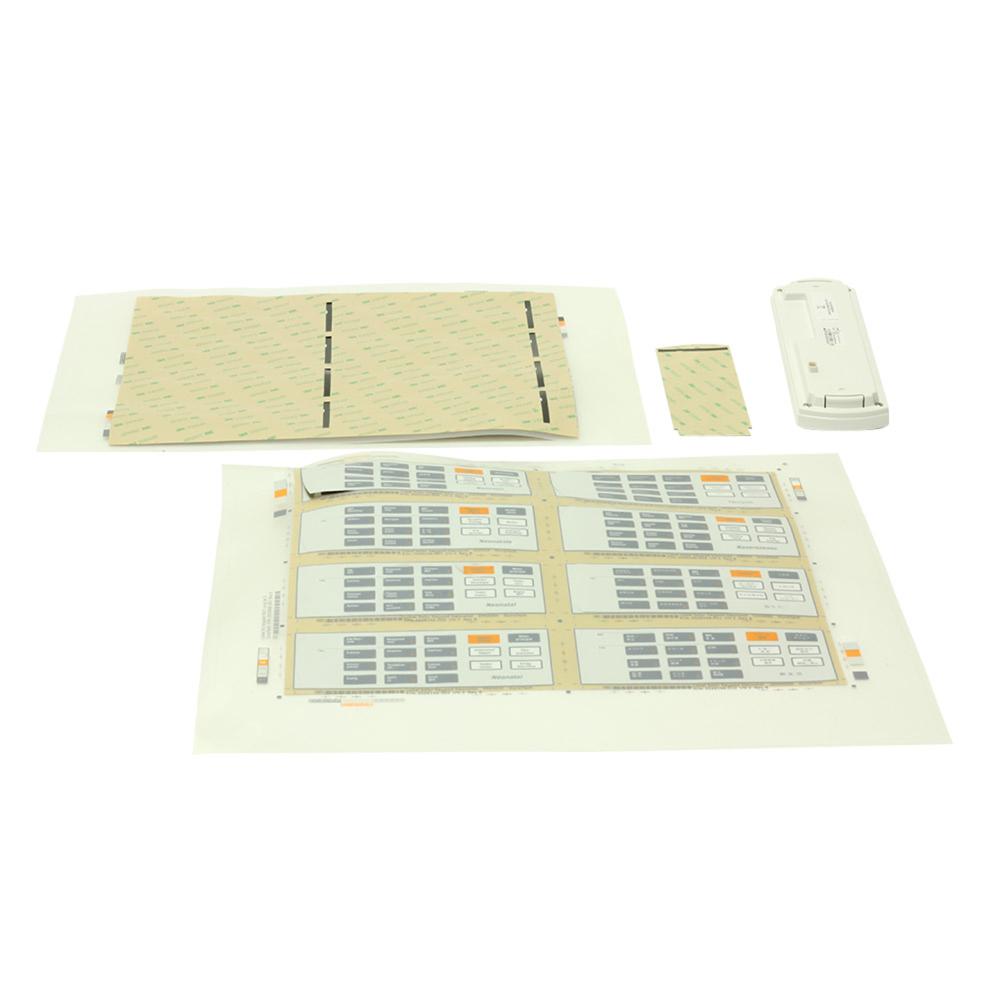
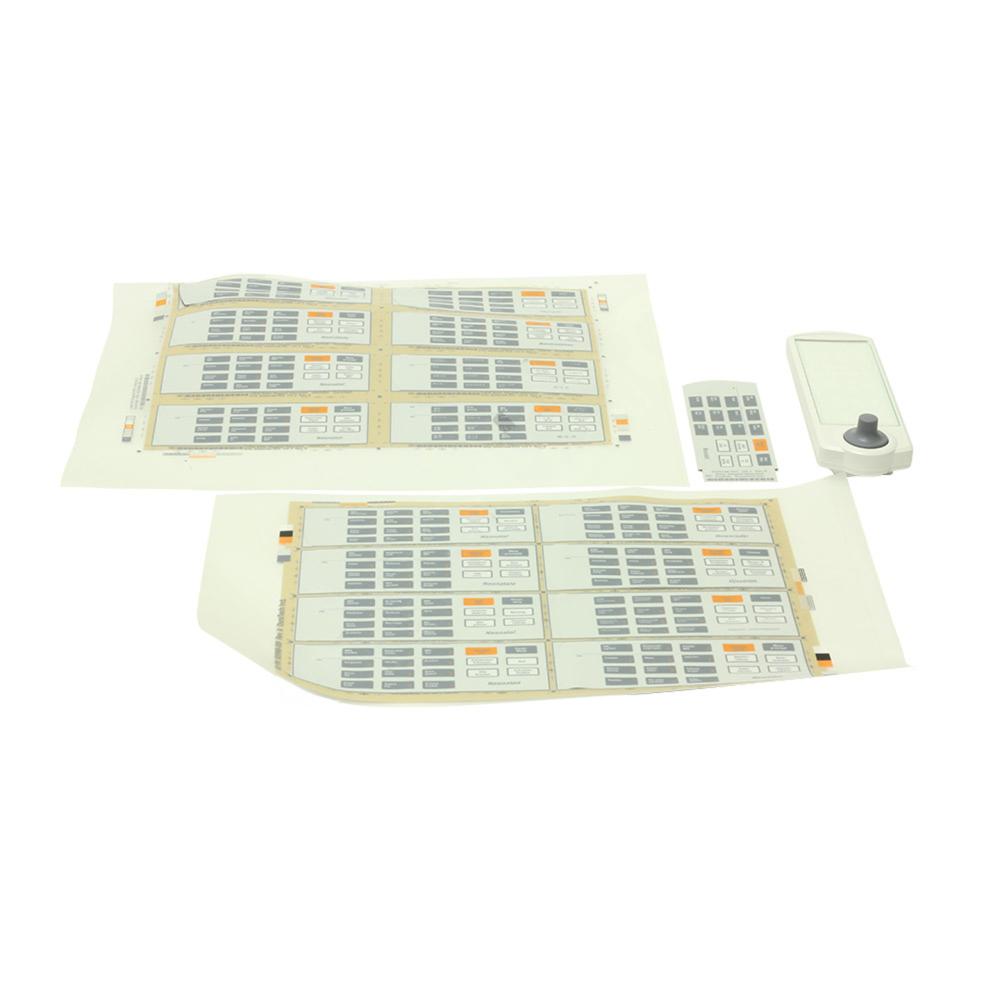
Kit Keypad Neonatal with Language Labels
| 2026967-003 | |
| 418708-901-1 | |
| New | |
| Patient Monitoring | |
| GE HealthCare | |
| GE Medical Systems Information Technologies | |
| GE HealthCare | |
| N/A | Outright |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- Clean and free from dirt, tarnish, oil, corrosive products, grease, lubricants and other contaminants
- Labels are to be die cut to liner with breakaway tab
- Label material and ribbon complies to UL 969 specifications
- Field Replacement Unit (FRU) - can be removed or replaced quickly and easily