Flapper Valve BCG 10.2 DIA x 3.9L Viton MPOS
| 1009-3097-000 | |
| New | |
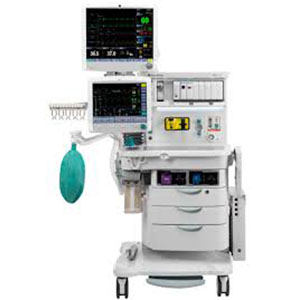
| Anesthesia Delivery | |
| GE HealthCare | |
| Datex-Ohmeda | |
| GE HealthCare | Outright |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- Cleaned to specific levels of hydrocarbon agents (i.e., oils, grease, etc.), particulates (dust, dirt, lint, fibers, paper, rust, chips, etc.) and other foreign materials (cleaning solutions and other coolants) on the surface or in the internal passages.
- Confines to standards like ISO 15001:2010(E) - Anesthetic and Respiratory Equipment - Compatibility with Oxygen
- Does not contain Cadmium (Cd) more than 0.01% by weight and Lead (Pb), Mercury (Hg), Hexavalent Chromium (Cr+6), Poly Brominated Biphenyl (PBB), Poly Brominated Diphenyl Ethers (PBDE), Bis(2-ethylhexyl) phthalate (DEHP), Butyl Benzyl Phthalate (BBP), Di Butyl Phthalate (DBP) and Di Iso Butyl Phthalate (DIBP) more than 0.1% by weight
- High chemical resistance